CNN
—
When docs informed Katrina Barry that she had a uncommon and critical situation known as pulmonary arterial hypertension or PAH, they warned her to not Google it.
Come on, she thought; they wished a younger lady who was certain for graduate faculty, who had survived a transatlantic aircraft flight whereas having a coronary heart assault and now open-heart surgical procedure, to not search for the situation that saved attempting to kill her?
Ready for her on the web was some chilling data. PAH impacts about 500 to 1,000 People every year, usually ladies between the ages of 30 and 60, according to the American Lung Affiliation.
Barry, who was 25 on the time, discovered from her studying that she had two to 5 years to dwell, primarily based on how extreme her situation already was.
Then her medical crew provided her a possible lifeline: a first-of-its-kind experimental drug known as sotatercept that corrals a progress issue that’s overproduced by folks with PAH, probably altering the underlying biology of the illness.
She signed up for a research to check the remedy, given each three weeks by injection below the pores and skin.
She began remedy on the daybreak of the Covid-19 pandemic, in March 2020. Barry stated she diligently wore an N95 masks and two layers of clothes and scrubbed herself down after each journey to the hospital for her injections to keep away from getting Covid alongside the best way.
“I needed to go to the hospital as a result of I used to be dying if I didn’t get this drug,” she stated. “This was the one probability I had at survival.”
On Tuesday, the US Meals and Drug Administration approved the remedy that Barry has taken for 4 years, a drug she credit with holding her alive and permitting her to renew most of the actions she loved earlier than her analysis.
Merck, the corporate that manufactures the drug, stated it is going to be bought below the model identify Winrevair.
Barry, now 29, is a affected person guide for Merck however stated she has not acquired compensation for telling her story.
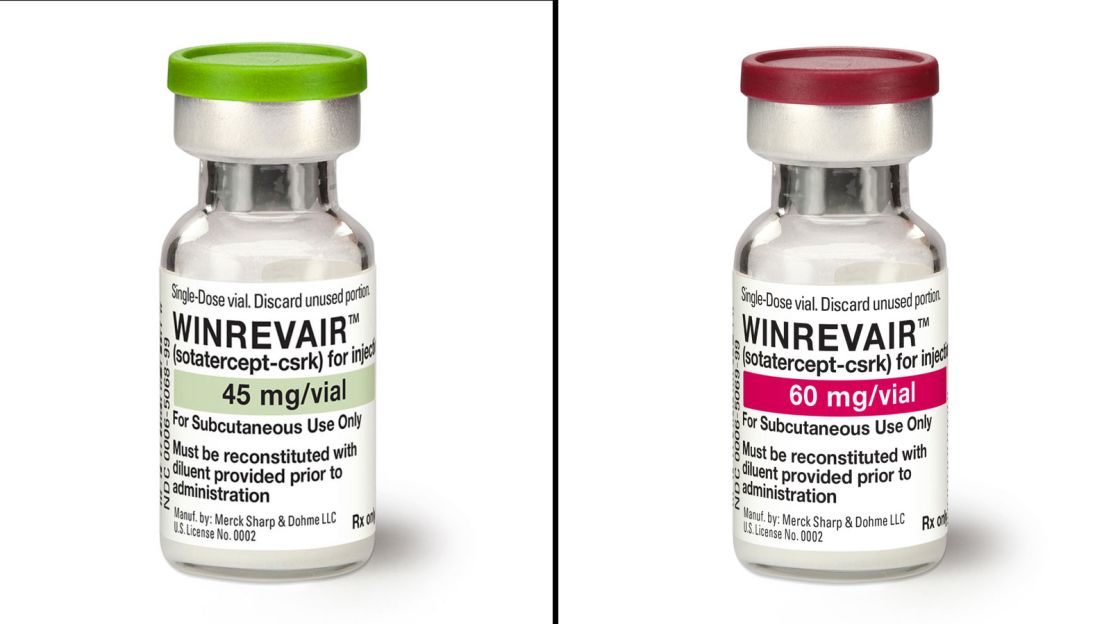
Winrevair is a biologic drug designed to seize onto and lure proteins known as activins which can be overproduced in PAH. These proteins trigger the partitions of an individual’s blood vessels to continue to grow and thicken over time.
Because the blood vessels slim, the guts is compelled to work tougher to pump blood to the lungs. This stress finally damages the guts, inflicting it to fail.
With out remedy, individuals who have PAH normally dwell about two to 3 years after their analysis. Therapy with a mix of medication that dilate, or chill out, blood vessels can enhance this outlook, however they don’t seem to be a remedy.
“This remedy has a a lot totally different mechanism of motion, and it could really rebalance a number of the progress that goes on in … the very small pulmonary arterioles the place the illness begins,” stated Dr. Vallerie McLaughlin, a heart specialist on the College of Michigan who helped research sotatercept.
“Which will assault the illness from a special angle and permit for what we returned as reverse transforming,” she stated, which means sufferers may really enhance over time whereas on the remedy.
Winrevair is the primary new kind of drug to come back alongside for sufferers with PAH in a protracted whereas, stated Dr. Panagis Galiatsatos, a pulmonary and demanding care drugs specialist at Johns Hopkins Bayview Medical Middle and a spokesperson for the American Lung Affiliation.
In animal research carried out earlier than the human trials, the drug regarded prefer it may do extra than simply deal with signs: It appeared prefer it may be capable of cease the thickening of the blood vessels and maybe lengthen sufferers’ lives, however these advantages haven’t been confirmed in people.
As a substitute, Galiatsatos stated, the FDA accredited the drug primarily based on different indicators of effectiveness, together with whether or not sufferers have been in a position to stroll farther in six minutes than they may earlier than they began taking the drug, a scientific measure known as the six-minute stroll take a look at.
In a research that included 323 individuals who took both sotatercept or a placebo for six months, those that obtained the drug have been in a position to stroll 34 meters – about half a metropolis block – farther in six minutes than they may earlier than they began remedy, whereas folks within the placebo group have been about to stroll solely about 1 meter greater than they may after they began the research. Each teams have been additionally taking the usual drugs for the situation, which assist chill out blood vessels to enhance blood move.
By the top of the research, 9 individuals, or about 5%, within the sotatercept group had died or had their illness worsen, whereas 42 sufferers, or about 26%, within the group taking a placebo had died or gotten worse.
The FDA was keen to behave quick to approve the remedy as a result of the illness, Galiatsatos stated, is a “ticking time bomb” for individuals who don’t have time to attend for longer research.
The research that led to the drug’s approval was published within the New England Journal of Medication final yr.
“Many people are excited a few remedy resembling this, so we are able to provide our sufferers one thing that we imagine is best than their present normal of remedy,” stated Galiatsatos, who famous that neither he nor the American Lung Affiliation has any monetary curiosity within the drug.
However Galiatsatos stated that as promising because the drug seems to be, there are nonetheless many unknowns, together with whether or not the drug will profit all PAH sufferers equally. Though a big variety of people who find themselves identified with the illness are African American, he famous, simply seven folks within the scientific trial – representing 2% of individuals – have been Black.
“I have a tendency to carry my breath” with any new drug, Galiatsatos stated, including that he “is ready for the real-world information to pour in to see how nicely this labored in additional numerous sufferers.”
In July 2019, Barry had rented an house for the summer season in Greece, the place she was wanting ahead to volunteering at a youngsters’s hospital. However whereas she was overseas, some shortness of breath that she had been asking docs about for extra than two years immediately worsened.
She wakened one morning gasping for air and realized the state of affairs was dire. She knew she needed to get again dwelling to Boston for assist. She known as her mother, they usually booked a flight for her again to the States.
She landed on the airport and went straight to the hospital. There, docs did a CT scan and located what regarded like dozens of blood clots within the tiny vessels that lace the lungs. Her coronary heart had been severely broken by a coronary heart assault.
“The heart specialist stated that my degree of coronary heart harm isn’t discovered till post-mortem,” Barry stated. “And each physician stated I shouldn’t have survived that aircraft trip dwelling.”
A fragile operation to take away the clots adopted – however the surgeons who opened her chest discovered that her blood vessels had really collapsed in lots of areas, mimicking blood clots. The blood stress inside her coronary heart was sky-high, and that’s after they lastly knew what they have been coping with PAH, she stated.
As a result of the illness is uncommon and sometimes impacts youthful ladies, delays in getting a analysis are sadly frequent, Barry stated.
As docs have been warming Barry’s physique to convey her out of surgical procedure, her coronary heart wouldn’t restart. She spent per week in an induced coma on a heart-lung bypass machine. The medical crew informed her mother and father that she would most likely want a direct heart-lung transplant.
After a number of days on the bypass machine, nevertheless, her physique started to recuperate, and docs have been in a position to convey her out of the coma.
When she wakened, she discovered about her pulmonary hypertension analysis.
At 25, going through a life far shorter than anticipated, Barry noticed all the things otherwise.
“Why am I saving if I’m not going to have a retirement?” she questioned. “Let me benefit from the life that I’ve now.”
However there wasn’t even a lot of life left to get pleasure from, she stated.
She was tethered to oxygen tanks and endlessly questioning what number of she would want for the period of time she wished to be out of the home. There was additionally a big new remedy pump that lived in a fanny pack she may by no means take off, even to bathe.
The pump delivered a steady stream of medication by way of a catheter threaded instantly into a big vein in her physique to assist preserve her blood vessels open. If one thing ever occurred to the pump, she had three hours to get to the closest hospital for assist, she was informed.
Being alive was good and all, Barry thought, however what about sporting a gown? What about going for a swim?
“The listing goes on of all of the issues I couldn’t do,” she stated.
Barry was identified shortly after her surgical procedure in October 2019, and she or he jumped on the probability to attempt sotatercept within the spring of 2020. The enhancements weren’t on the spot, however after a number of months, she went from not having the ability to even fold her laundry to having the ability to perform a little extra. She was in a position to go for brief hikes and kayak a bit.
After a yr, Barry stated, she was in a position to come off her steady oxygen remedy and use it solely at night time. She was finally in a position to cease utilizing it at night time, too.
After about 18 months, docs have been in a position to take away her central line and the cumbersome, ever-present med pump, permitting her to change from the IV variations of her vasodilating medicine to the capsule kinds.
“For me, that was so life-changing,” Barry stated.

She improved a lot, she was in a position to return to Greece in 2022 and hike up a volcano. Final month, she went snowboarding.
Barry nonetheless has PAH. Her lungs burn and she or he will get wanting breath whether or not she exerts herself loads.
“But it surely has gotten higher over time,” Barry stated.
She’s enrolled in a grasp’s diploma program in public well being, and she or he was in a position to put on a gown to her brother’s marriage ceremony. She has not wanted the heart-lung transplant that docs thought she may.
“It’s a miracle drug, actually,” she stated of sotatercept. “That sounds bizarre to say, nevertheless it gave a lot optimistic however not a lot detrimental.”
She does know a minimum of one particular person within the tightly knit PAH neighborhood who skilled bleeding whereas on the drug and needed to cease taking it.
Bleeding and dizziness among the many dangers
In scientific trials, folks on sotatercept had extra nosebleeds due to burst blood vessels of their noses, they usually developed extra spider veins below their pores and skin. Consultants say the drug could spur the expansion of latest blood vessels.
Different unwanted side effects included dizziness and elevated hemoglobin, the molecule that carries oxygen within the blood. Some folks with PAH have already got elevated hemoglobin ranges as their our bodies attempt to compensate for the situation, so including the drug on high of that is likely to be an excessive amount of for them, specialists stated.
Sotatercept also can lower platelets, the cells that assist type clots, which will increase the danger of bleeding.
“There’s been some stories of gastrointestinal bleeding and even bleeding inside the mind,” stated Dr. Kristin Highland, a lung specialist on the Cleveland Clinic who treats folks with PAH. “So bleeding might be an actual facet impact.”
Docs who’ve examined sotatercept imagine that it may very well be an enormous advance. However additionally they acknowledge that there are nonetheless numerous unknowns, resembling whether or not folks on sotatercept might want to preserve taking the cocktail of medication that assist to chill out their blood vessels and decrease their blood stress, Highland stated.
“That’s the million-dollar query,” she stated. “Definitely, there’s numerous hope that perhaps this is able to permit decrease doses or presumably a type of therapies to be eliminated. However I don’t assume we fairly have the reply to that.”
Merck says it plans to cost a wholesale price of $14,000 per vial and dosing relies on a affected person’s weight. The remedy will are available in single or double vial kits that can be utilized at dwelling.
Primarily based on information from scientific trials, Merck says, it expects that the majority sufferers will want a single vial each three weeks, for an annual price of about $243,000.
The wholesale price isn’t usually what sufferers pay. The ultimate price to people is set after insurance coverage suppliers negotiate the value.
Nonetheless that’s considerably greater than some analysts had hoped to see. The nonprofit Institute for Medical and Financial Evaluate stated the advantages of the drug could be price the fee at around $35,000 per yr.
Merck stated in a information launch that it anticipated the drug to be in specialty pharmacies by the top of April.
At the least initially, sufferers should get their injections of sotatercept in a clinic, the place they are often monitored. Over time, Barry stated, she was in a position to start giving herself the injections at dwelling, which has made it extra handy.
Dr. Aaron Waxman, Barry’s physician and the director of the pulmonary vascular illness program at Brigham and Girls’s Hospital in Boston, stated he’s seen different sufferers who’ve carried out in addition to she has within the six years they’ve been finding out the drug. These folks have additionally been in a position to get off their med pumps and change to drugs for his or her different therapies.
One such capsule – Opsynvi, a brand new mixture of two generally taken medicine made by Johnson & Johnson – obtained FDA approval on Monday to deal with PAH.
“We’re seeing marked enchancment that will recommend reversal” of illness with sotatercept, Waxman stated.
“I’ve by no means seen this type of enchancment within the illness earlier than,” he stated. “I feel this can be a game-changer.”
Barry says the drug has already been a beacon for individuals who could not have a lot to struggle for.
“It’s introduced a lot hope to individuals who who’ve misplaced all hope due to this deadly illness,” she stated.