CNN
—
For her total life, school scholar Olivia Prepare dinner had solely a small diploma of central imaginative and prescient. It was as if she was watching the world by a straw gap, and in dimly lit locations, she couldn’t make out folks’s faces, solely their silhouettes.
However after receiving an experimental gene-editing therapy to one in every of her eyes, she now can see issues she by no means noticed earlier than.
Prepare dinner was born with an inherited retinal dysfunction that causes blindness, a uncommon kind of eye dysfunction traditionally referred to as Leber congenital amaurosis or LCA. A number of years in the past, she determined to take part in a medical trial that concerned utilizing the gene-editing device CRISPR to appropriate the type of inherited blindness that she has.
“My life has principally modified when it comes to being hopeful that there’s going to be extra science and findings sooner or later,” mentioned Prepare dinner, 22, who’s at present finding out advertising and product growth at Missouri State College in Springfield. She acquired the experimental gene-editing therapy by a surgical procedure carried out on her left eye.
“Now, post-surgery and submit restoration, I’m able to see in dimmer lighting with my left eye,” Prepare dinner mentioned.
A therapy that used CRISPR was discovered to be secure and efficacious in enhancing imaginative and prescient amongst a small pattern of sufferers with inherited blindness within the Section 1/2 medical trial that Prepare dinner participated in. Inherited retinal degenerations are a number one explanation for blindness all over the world.
Amongst a complete of 14 volunteers, together with Prepare dinner, the gene-editing device was discovered to be related to a “significant enchancment” in imaginative and prescient for many sufferers round three months later and it was circuitously tied to any severe uncomfortable side effects, in line with the trial outcomes, printed Monday within the New England Journal of Drugs. The remedy stays experimental and the outcomes must be replicated in a bigger group of individuals.
Months following the therapy, Prepare dinner was sitting with associates on a balcony that had Christmas lights wrapped across the railing. It was nightfall, she recalled, but she may see her associates’ faces glow beneath the twinkling Christmas lights. She was shocked.
“With my proper eye, I used to be not in a position to see their facial options. I used to be solely in a position to see their silhouette. With my left eye, I may see the whole lot on their face – so, important distinction, particularly within the dim lighting,” Prepare dinner mentioned about that night.
“One of many greatest ‘aha moments’ that I had was I had been speaking to my mother sooner or later after the surgical procedure – it was about six to 9 months after the surgical procedure after I seen most of my enchancment,” Prepare dinner mentioned.
“I may see a candle flickering behind me, which I’ve by no means seen that earlier than,” she mentioned. “I’d by no means picked up something from over there earlier than with the peripheral.”

Earlier than the therapy, Prepare dinner mentioned that she typically may conceal the imaginative and prescient challenges she has had. Her restricted imaginative and prescient typically was an inner battle.
“You wouldn’t actually know that my eyesight is horrible till you spend a big period of time with me,” Prepare dinner mentioned. “If we noticed one another on the street, if I launched myself to you, you’d by no means know.”
However now, she is now not hiding.
This examine is the primary time that CRISPR has been used within the eyes of dwelling folks.
“The outcomes of this examine present proof of idea that CRISPR-Cas9 gene modifying can be utilized safely and successfully to deal with inherited retinal problems,” mentioned the examine’s first creator Dr. Eric Pierce, director of the Ocular Genomics Institute at Mass Eye and Ear and Harvard Medical Faculty.
The trial was funded by the biotechnology firm Editas Drugs and carried out in america by researchers at Mass Eye and Ear of the Mass Basic Brigham well being care system and different US-based establishments, together with the Perelman Faculty of Drugs on the College of Pennsylvania, the College of Michigan, the College of Miami, and Oregon Well being & Science College.
“We’re actually hopeful that CRISPR-Cas9 gene modifying applied sciences will now be utilized to different genetic types of inherited blindness, and certainly different genetic ailments basically,” Pierce mentioned. “We’re hoping this may assist open the period of therapeutic use of CRISPR-Cas9 applied sciences.”
The trial, which began in 2019, enrolled 12 adults, ages 17 to 63, and two kids, ages 9 and 14, with inherited retinal degeneration brought on by mutations within the CEP290 gene. That gene offers directions for making a protein concerned in lots of forms of cells, together with gentle receptor cells within the eyes. Mutations in CEP290 are the most typical explanation for extreme early-onset retinal degeneration, which causes imaginative and prescient loss in kids.
At the moment, there isn’t any therapy authorised by the US Meals and Drug Administration for CEP290-associated inherited retinal degeneration. These sufferers wouldn’t be capable of learn any strains of letters or numbers on a imaginative and prescient chart that most individuals obtain on the eye physician, and visible impairment might worsen over time.
For the trial, the 14 contributors underwent a surgical process through which a drug referred to as EDIT-101 that encodes the CRISPR gene-editing elements was injected beneath the retina of one in every of their eyes. Because the trial was carried out to primarily consider security and efficacy, just one eye in every affected person was studied.
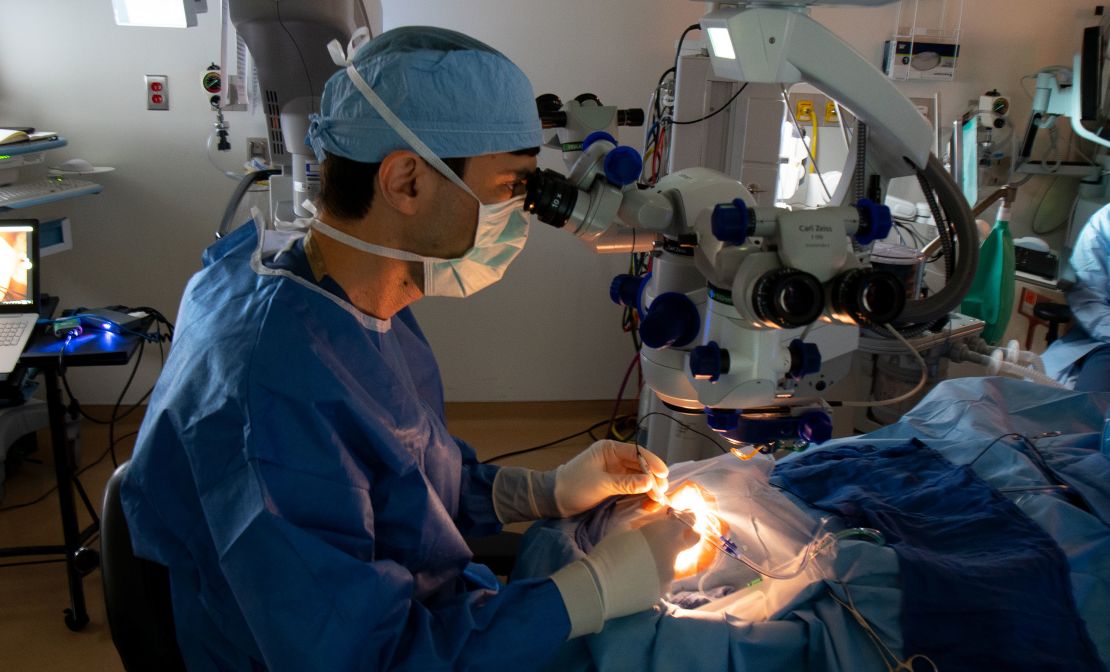
“The themes get an injection of the gene-editing drug, which known as EDIT-101, beneath their retina,” Pierce mentioned. “That drug encodes the CRISPR-Cas9 gene-editing equipment, and as soon as that begins working contained in the retinal cells of these sufferers, it cuts out the mutation in CEP290 from the genome of their retinal cells, permitting the perform of the CEP290 gene to be restored.”
When the primary sufferers within the examine had been handled in 2020, it was the first time in medical history that a CRISPR-based medicine, resulting in gene-editing, was inserted immediately into the dwelling human physique.
Among the many grownup volunteers, two got a low dose of the treatment, 5 got an intermediate dose and 5 got a excessive dose. Each of the kids within the examine got the intermediate dose. The outpatient process took round an hour and a half.
The sufferers had been then monitored each three months for a yr after which much less frequent monitoring continued for 2 years. In these follow-up visits, they underwent a collection of imaginative and prescient assessments amongst different evaluations.
The researchers discovered that 11 sufferers within the examine had some kind of enchancment of their imaginative and prescient following the CRISPR remedy, and these enhancements occurred about three months after the process and had been sustained throughout subsequent visits.
Additionally, no severe uncomfortable side effects occurred in response to the therapy at any of the dose sizes, in line with the researchers, and the adversarial occasions that did happen had been gentle or reasonable. There have been additionally no indicators that the CRISPR gene-editing induced ripple-effect hurt to the genomes of the sufferers.
“The first purpose of this primary in-human examine was to check the security of utilizing CRISPR-Cas9 gene modifying in vivo. After we began the trials, the themes who had been handled had been the primary sufferers ever to have acquired CRISPR-Cas9 gene-editing remedies in vivo,” Pierce mentioned. “There have been no severe adversarial occasions associated to the therapy, or the surgical procedure required to ship the therapy and no dose-limiting toxicities.”
Following the surgical procedure, one affected person skilled some bleeding within the eye, impairing their imaginative and prescient, however that has since resolved, in line with the researchers.
“As soon as that hemorrhage cleared, the topic’s imaginative and prescient returned to baseline,” Pierce mentioned.
One other affected person skilled imaginative and prescient impairment related to small mounds noticed beneath their retina six months after the process. These kinds of hyperreflective mounds have been seen in different research involving subretinal gene therapies, the researchers famous, and the reason for them is just not clear.
“It’s regarded as irritation,” Pierce mentioned in regards to the mounds.
The affected person was handled with a course of steroid medicines, in line with the examine, and their restoration is ongoing.
“Because the mounds resolved their imaginative and prescient additionally improved,” Pierce mentioned. “I believe this drug was as secure as potential when it comes to design.”
Full imaginative and prescient has not been restored among the many sufferers. Most within the trial couldn’t learn any line of an eye fixed chart previous to the examine, and solely 4 of them skilled some enhancements on this potential. However some sufferers reported, after receiving therapy, with the ability to see their cell telephones gentle up, differentiate numerous meals on their dinner plates, establish the spinning Apple icon on a pc display screen and even noticing vibrant sunsets.
“I began to see what are described as bursts of shade,” mentioned Michael Kalberer, 46, who received the CRISPR treatment in his right eye and first seen enhancements in his imaginative and prescient about two to 6 months later. He began the examine in 2020.
“It was a reasonably cool second to see strobe lights on the dance ground of my cousin’s marriage ceremony change shade,” mentioned Kalberer, who added that if he had not acquired the therapy, all he would have seen on the dance ground would have been shadows and flickering lights, and he wouldn’t have been in a position to establish the colours.
Kalberer described the CRISPR therapy as “groundbreaking,” however warned it’s not a remedy.
“It’s not a panacea,” mentioned Kalberer, who nonetheless can’t see normal textual content or photographs on a display screen. “My illness remains to be right here. It’s not gone. I’m not cured. … However it undoubtedly slowed the development of it.”
Pierce mentioned that he hopes this method to utilizing CRISPR as a remedy for inherited blindness might be studied once more in a bigger and extra numerous group of sufferers. The entire Section 1/2 trial contributors had been non-Hispanic and White.
In 2022, Editas Medicine announced that it paused additional finding out CRISPR gene modifying as a therapeutic method for CEP290-associated inherited blindness and as an alternative of conducting additional trials, has continued to follow-up with the sufferers who’ve been handled to this point.
The newest outcomes from the Section 1/2 trial assist transferring ahead with a Section 3 trial after which in the end registering the remedy for potential FDA approval, Pierce mentioned.
“We’re working with Editas to establish a further business companion for Section 3 research. We’re truly hoping this publication will stimulate curiosity within the biotech and pharma communities about that,” Pierce mentioned.
Extra analysis over time may make clear the long-term results of the CRISPR-Cas9 gene modifying instruments, which, now that they’ve been injected into sufferers, might be current in sufferers for the remainder of their lives, Pierce mentioned.
“I believe the actual danger that we’re all involved about with CRISPR-Cas9 gene modifying is: Might the gene modifying equipment that we’ve launched into the retinal cells of those sufferers do one thing else, some place else within the genome, along with the therapeutic actions that it was designed for?” Pierce mentioned.
“Might a minimize within the genome be made 10 years from now, that might have an adversarial impact over time? I believe the reply to that’s sure, it may. However we’re hopeful that danger could be very low,” Pierce mentioned. “That’s what we’d like further follow-up for.”
The outcomes from the Section 1/2 trial — and the way sufferers skilled some enhancements in imaginative and prescient — are a beneficial reminder of how essential high quality of life might be for sufferers, mentioned Artwork Caplan, a professor of bioethics and founding head of the Division of Medical Ethics at NYU Grossman Faculty of Drugs’s Division of Inhabitants Well being.
“Normally once we’re doing gene therapies or different modern interventions, we affiliate them with saving lives. This experiment is a big reminder that high quality of life issues. That is about imaginative and prescient,” Caplan mentioned. “Nobody’s dying. Nobody’s saved. However restoration of imaginative and prescient is a crucial achievement, and it’s a reminder that high quality of life needs to be factored into what we determine to cowl when it comes to insurance coverage, reimbursement and what we attempt to examine.”
He agreed with the researchers that extra security knowledge over time can be useful.
“They haven’t actually had these topics that lengthy with the intervention to ensure long-term security,” Caplan mentioned. “For these sorts of genetic interventions, you must comply with them over lengthy durations of time — years — to guarantee that different genes weren’t impacted.”
These new Section 1/2 trial outcomes present a “constructing block” for scientists to work off of sooner or later when growing gene therapies to deal with eye problems, mentioned Dr. Vlad Diaconita, a retinal surgeon and assistant professor of ophthalmology at Columbia College Vagelos School of Physicians and Surgeons. He was not concerned within the trial.
“Does this apply to the American inhabitants at massive? Not proper now,” Diaconita mentioned in regards to the experimental therapy.
“It does, nonetheless, apply to the hundreds of children born in future years which have this explicit genetic subtype. So sure, an approval of this explicit gene supply may gain advantage folks over time,” he mentioned. “It’s a proof of idea that appears to be transferring us in the proper path.”
Diaconita’s colleague Dr. Aliaa Abdelhakim referred to as this proof-of-concept examine “groundbreaking” within the sense that it exhibits the therapy method might be secure and lead to some enchancment for sufferers, however extra analysis is required on a bigger scale to find out what sort of sufferers will profit in the long run, and the way lengthy these enhancements might final.
“We nonetheless have to attend just a little longer to see if this pans out within the long-term,” mentioned Abdelhakim, an ophthalmologist-geneticist, retina specialist and assistant professor of ophthalmology at Columbia College Vagelos School of Physicians and Surgeons. She additionally was not concerned within the trial.
“We don’t know if enhancements from this therapy are going to be sustained. Is their imaginative and prescient going to keep improved all through their lives?” she requested. “The rationale that is essential is as a result of that is the primary time CRISPR has been used on this method, within the eye.”